Kinase-squelching mutants of chemoreceptor signaling fragments by Peter Ames, Luisa Capalbo and Sandy Parkinson |
|
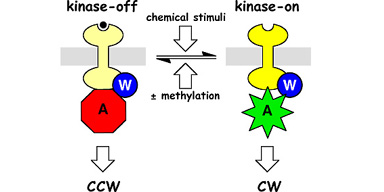
| Bacterial chemoreceptors like Tsr, which mediates chemotaxis toward serine, have two signaling states (Fig. 1). In the kinase-on state, the receptors stimulate the activity of CheA, a histidine protein kinase, to enhance clockwise (CW) rotation of the flagellar motors. In the kinase-off state, the receptors inhibit or squelch CheA activity to augment counter- clockwise (CCW) flagellar rotation. The cell's locomotor behavior is regulated by the proportions of receptors in these two signaling states. The equilibrium distribution of receptor signaling states is regulated by chemoeffectors (e.g., serine) and by reversible covalent modifications (methylation), enabling the cell to follow spatial gradients of attractant and repellent chemicals in its environment. | |
Figure 1. Two-state model of chemoreceptor signaling. Chemoreceptors of the MCP (methyl-accepting chemotaxis protein) family form ternary signaling complexes with the cytoplasmic CheW and CheA proteins. Receptor methylation and ligand occupancy drive an equilibrium between kinase-stimulating and kinase-squelching signaling states to control the cell's swimming behavior. |
 |
| When we "liberated" the signaling domain of Tsr from its ligand-binding domain and methylation sites, the resulting soluble fragments exhibited kinase-on behavior (see related poster). To study the kinase-off signaling state, we devised behavioral screens and selections to look for mutants of a CW-signaling Tsr fragment that were locked in the CheA-squelching conformation. Only a few specific mutational changes were able to cause squelching (Fig. 2, left). We purified several squelching fragments and found that they inhibited CheA activity by about 80% in vitro. Moreover, the CheW coupling protein was not required for this effect, demonstrating that receptors in the kinase-off state make direct, reversible contact(s) with the CheA molecule. To locate the CheA contact region in the receptor, we trimmed superfluous residues from a squelching Tsr fragment. Our smallest CheA-squelching receptor fragment is nearly identical in length to our smallest CheA-stimulating fragment, suggesting that the same CheA contact site(s) may participate in both signaling activities (Fig. 2, right). | |
Figure 2. CheA-squelching Tsr fragments. (Left) Alpha-carbon backbone of the Tsr signaling domain. The space-filled amino acids indicate residues at which mutational changes can lock the receptor in a kinase-squelching conformation. (Middle) Space-filling view of the intact Tsr dimer. (Right) Minimal CheA-squelching fragment. The mutant residue that locks this fragment in the squelching conformation (A413V; black) lies at the interface between the two subunits and is mostly buried (see cross- section view). |
 |
| Tsr fragments carrying the A413V mutation migrate more slowly than their wild-type counterparts upon electrophoresis in nondenaturing polyacrylamide gels, suggesting that receptors in the CheA-squelching conformation are less flexible than those in the CheA-stimulating conformation. Consistent with this view, the A413V replacement was found to enhance the stability of the dimeric form of receptor fragments, possibly by strengthening hydrophobic packing interactions at the dimer interface (see Fig. 2). We hope to exploit these kinase-squelching Tsr fragments to learn more about the structure of the kinase-off conformation and the mechanisms of chemoreceptor signaling. | |
| Ames, P. & J.S. Parkinson (1994) Constitutively signaling fragments of Tsr, the E. coli serine chemoreceptor. J. Bacteriol. 176: 6340-6348. | |
